David Kistenbroker, Joni S. Jacobson and Angela M. Liu are Partners at Dechert LLP. This post is based on a Dechert memorandum by Mr. Kistenbroker, Ms. Jacobson, Ms. Liu, Christine Isaacs and Biaunca S. Morris.
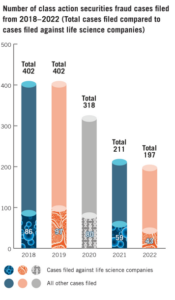
In 2022, the total number of securities class action complaints filed remained below the more elevated levels we saw during 2017-2020, but life sciences companies were nonetheless still popular targets among these filings. [1] In this post, we analyze and discuss trends identified in filings and decisions from 2022 so that prudent life sciences companies can continue to take heed of the results.
Plaintiffs filed a total of 43 securities class action lawsuits against life sciences companies in 2022, which represented almost one in four securities class action lawsuits. Filings against life sciences companies in 2022 represented a 27.1% decrease from the previous year, and a 51.1% decrease from five years prior. Of these cases, the following trends emerged:
- Consistent with historic trends, the majority of suits were filed in the Second, Third and Ninth Circuits, with a 54.5% decrease in suits filed in the Ninth Circuit – 22 in 2021 and 10 in 2022. The Third Circuit saw a 44.4% decrease in filings from the previous year – from nine in 2021 to five in 2022. For district courts within these circuits, the Southern District of New York had the most filings, with 10 overall
- A few plaintiff law firms were associated with about three-fourths of the first filed complaints against life sciences companies: Pomerantz LLP (18 complaints), Glancy Prongay & Murray LLP (five complaints) and Bronstein, Gewirtz & Grossman, LLC and Kessler Topaz Meltzer & Check LLP (tied with four complaints each). [2]
- Slightly more claims were filed in the first half of 2022 than in the second half, with 24 complaints filed in the first and second quarters, and 19 complaints filed in the third and fourth quarters.
- About a quarter of the securities fraud cases brought against life sciences companies (11 cases) were filed against companies with COVID-19-related products and services.
An examination of the types of cases filed in 2022 reveals continuing trends from previous years.
- About 48.8% of claims, or 21 of 43 claims, involved alleged misrepresentations regarding product efficacy and safety, [3] with many of these cases involving alleged misrepresentations regarding certain negative side effects associated with leading product candidates, which could potentially impact the likelihood of Food and Drug Administration (“FDA”) approval.
- About 39.5% of the claims, or 17 of 43 claims, arose from alleged misrepresentations regarding the sufficiency of the applications submitted to the FDA.
- Approximately 20.9% of the claims, or nine of 43 of claims, alleged misrepresentations regarding purported unlawful conduct in both the United States and abroad, including (but not limited to) illegal kickback schemes, criminal investigations and inadequate internal controls in financial reporting.
- About 25.6% of the claims, or 11 of 43 claims, involved alleged misrepresentations of material information made in connection with proposed mergers, sales, initial public offerings (“IPOs”), offerings and other transactions. [4]
Courts throughout the country issued decisions in 2022 involving securities fraud actions against life sciences companies, including:
- Claims that arose in the development phase, such as cases involving products failing clinical trials that are required for FDA approval or products not approved by the FDA. In these development phase cases, courts were more likely to grant motions to dismiss in full than they were to deny them, either in whole or in part.
- Claims that were independent of or arose after the development process. In these post-development cases, courts were more likely to grant motions to dismiss in full than they were to deny them, either in whole or in part.
- Claims based on the financial management of life sciences companies. In these cases, courts were more likely to deny them in part.
Given the numbers from 2022 and recent years’ filings, and accounting for residual impact of the COVID-19 pandemic, there is no indication that the filings of securities claims against life sciences companies are going to slow down any time soon, and plaintiffs continue to have mixed results in surviving a motion to dismiss. The decisions in 2022 resulted in a variety of outcomes, with 21 opinions decided in favor of defendants, [5] 10 opinions [6] denying motions to dismiss and 11 opinions in which only partial dismissal was achieved. [7] These numbers illustrate how life sciences companies remain attractive targets for class action securities fraud claims. Therefore, companies should continue to stay abreast of recent developments and implement best practices to reduce their risk of being sued.
Download the full report here.
Endnotes
1In 2022, 197 securities class actions were filed. Cornerstone Research, Stanford Univ., Securities Class Action Clearinghouse: Filings Database, SECURITIES CLASS ACTION CLEARING HOUSE (last visited Feb. 6, 2023). In 2020, 318 securities class actions were filed while 211 were filed in 2021. Id. Cornerstone Research reported 208 class action filings in 2022 and 218 in 2021, which included filings in both federal and state courts. Securities Class Action Filings 2022 Year in Review, Cornerstone Research, 1 (2023), https://www.cornerstone.com/wp-content/uploads/2023/01/Securities-Class-Action-Filings-2022-Year-in-Review.pdf(go back)
2These figures are based on the first complaint filed.(go back)
3This category also includes any issues at clinical trial. This category does not include deficiencies at the manufacturing site, nor are product deficiencies that arise from the deficiencies at the manufacturing site included in this category.(go back)
4It should be noted that 79.1%, or 34 of 43 claims, of all 2022 filings fell in more than one category.(go back)
5Throughout this post, the terms “company” or “defendants” may be used to also include individual officers or directors.(go back)
6This includes two cases where the motions were denied as moot because of settlements. See In re Sesen Bio, Inc. Sec. Litig.,No. 21-cv-7025 (S.D.N.Y. Aug. 31, 2022); Patrick McDermid v. Inovio Pharm.s, Inc. et al., 20-cv-1402 (E.D. Pa. Aug. 31, 2022).(go back)
7The opinions were identified by evaluating the dockets of “Healthcare” filings from 2020 and 2021 and reviewing the docket for a disposition decision taking place in 2022. Additionally, opinions were also identified through Westlaw searches of dispositive orders involving the Private Securities Litigation Reform Act (“PSLRA”) between January 1 and December 31, 2022 and cross-referencing them against filters in the Securities Class Action Clearinghouse filings by “Healthcare.” They may not encompass all dispositive opinions. In many cases, the court dismissed the operative complaint without prejudice and amended complaints are anticipated.(go back)
 Print
Print